this post was submitted on 17 Nov 2023
578 points (96.9% liked)
Science Memes
15755 readers
3131 users here now
Welcome to c/science_memes @ Mander.xyz!
A place for majestic STEMLORD peacocking, as well as memes about the realities of working in a lab.

Rules
- Don't throw mud. Behave like an intellectual and remember the human.
- Keep it rooted (on topic).
- No spam.
- Infographics welcome, get schooled.
This is a science community. We use the Dawkins definition of meme.
Research Committee
Other Mander Communities
Science and Research
Biology and Life Sciences
- !abiogenesis@mander.xyz
- !animal-behavior@mander.xyz
- !anthropology@mander.xyz
- !arachnology@mander.xyz
- !balconygardening@slrpnk.net
- !biodiversity@mander.xyz
- !biology@mander.xyz
- !biophysics@mander.xyz
- !botany@mander.xyz
- !ecology@mander.xyz
- !entomology@mander.xyz
- !fermentation@mander.xyz
- !herpetology@mander.xyz
- !houseplants@mander.xyz
- !medicine@mander.xyz
- !microscopy@mander.xyz
- !mycology@mander.xyz
- !nudibranchs@mander.xyz
- !nutrition@mander.xyz
- !palaeoecology@mander.xyz
- !palaeontology@mander.xyz
- !photosynthesis@mander.xyz
- !plantid@mander.xyz
- !plants@mander.xyz
- !reptiles and amphibians@mander.xyz
Physical Sciences
- !astronomy@mander.xyz
- !chemistry@mander.xyz
- !earthscience@mander.xyz
- !geography@mander.xyz
- !geospatial@mander.xyz
- !nuclear@mander.xyz
- !physics@mander.xyz
- !quantum-computing@mander.xyz
- !spectroscopy@mander.xyz
Humanities and Social Sciences
Practical and Applied Sciences
- !exercise-and sports-science@mander.xyz
- !gardening@mander.xyz
- !self sufficiency@mander.xyz
- !soilscience@slrpnk.net
- !terrariums@mander.xyz
- !timelapse@mander.xyz
Memes
Miscellaneous
founded 2 years ago
MODERATORS
you are viewing a single comment's thread
view the rest of the comments
view the rest of the comments
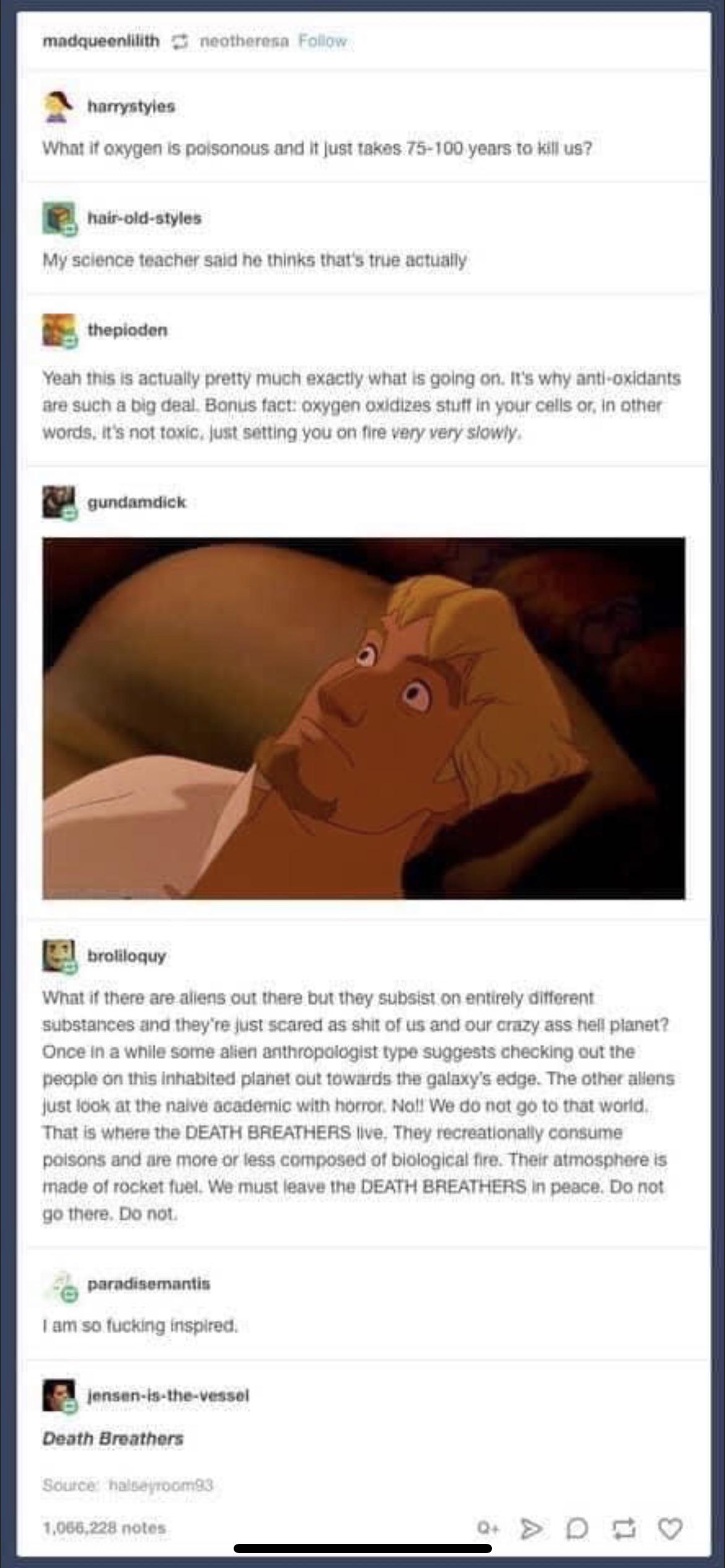
We keep finding life on earth in places where we didn't think life was possible. And yet, when we look at the stars we have the nerve to talk about there being a "goldilocks zone" for planets in other solar systems, like that's the only way life could exist there.
I'm sure there's life out there somewhere, and I wouldn't be surprised at all if carbon/water based life turns out to be a minority in the universe.
Yes, water is a simple compound made of some of the most common elements in the universe, so it's reasonable to think other life might also evolve to use it. Carbon is also a really handy element for making complex molecules, and is also really common. But, it's a failure of the imagination to think that life elsewhere has to follow the same basic chemistry as here on earth.
Unfortunately, most life will likely be Carbon based, in some manner (synthetic life could be different). The key is forming the large, complex molecules that make life, life. You need an element that can form chains. You also need to attach things to those chains.
The only 2 atoms that can do this are carbon and silicon. Both can form "organic" type molecules. Unfortunately, silicon has an additional reaction pathway that makes the chain easily break down in the presence of water. The conditions for silicon based life are so odd as to be unlikely to happen on the scale needed by natural processes. There might be some work arounds we don't know about, they would be extremes.
Synthetic life is another story. Once you have active control over your environment, a number of other options open up. The first step is the kicker however, getting from abiotic natural rubble to a working replicator.
There's a reason we are looking in Goldilocks zones, they are the most likely environment for the only process that seems viable.
Man, I love science people. Like, are you guys aware of how cool you are? I love being around people who talk about awesome shit like this, even tho I don't understand most of it. Keep being you o7